ADINA Act — inspired by Minnesota girl's allergy scare — would label drug ingredients
ADINA Act would label drug ingredients
A bill introduced in Congress that was inspired by a Maple Grove seventh-grader would require drug makers to label the ingredients on their products. FOX 9’s Courtney Godfrey has the story behind the ADINA Act.
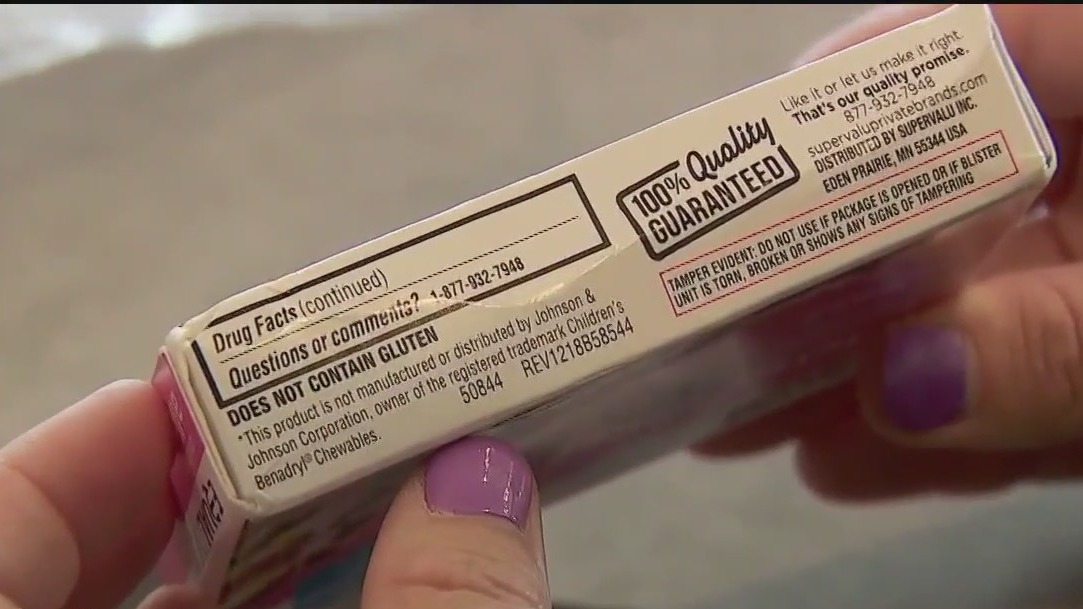
MAPLE GROVE, Minn. (FOX 9) - A Maple Grove seventh-grader is pushing for better labels on prescriptions and over-the-counter medications, after having a severe reaction to an unlisted food allergen in a common antibiotic.
Adina Togal was at an overnight camp last summer when she had a severe reaction to an unlisted allergen in the Amoxicillin pill she was prescribed. Her mother says they contacted the drug manufacturer, but the company refused to release a list of potential allergens without a letter from a licensed physician. Weeks passed before they received what they were looking for, and she says even then the allergens weren’t clear.

A Maple Grove seventh-grader is pushing for better labels on prescriptions and over-the-counter medications, after having a severe reaction to an unlisted food allergen in a common antibiotic. (FOX 9 / FOX 9)
"It shouldn’t be that hard to make sure the medication you’re taking is safe for you," said Jennifer Togal.
The 12-year-old has Celiacs disease along with a dairy allergy. Even the smallest amount of gluten prompts an intense physical reaction that includes vomiting, fatigue and brain fog. Her mother says while the pill she took was small, it was one of the worst food allergy reactions Adina has ever had.
"She was throwing up and in the middle of throwing up she passed out. Her medical team said her heart rate is dropping — she’s not stable — she can’t communicate with us and she’s in a lot of abdominal pain," Jennifer recalled.
Her experience has prompted Adina to pursue enacting federal legislation that would require drug companies to list the presence of the top nine allergens on medication labels. The bill has the support of major medical and allergy advocacy organizations, including FARE (Food Allergy Research and Education).
U.S. Rep. Dean Phillips, D-Minn., is the chief author of the bill. He says when the Togals came to him, he was shocked to learn that medicines do not have the same allergen labeling requirements that apply to food items.
"It's not about changing the process or putting a burden on the pharmaceutical manufacturers, other than simply putting on the label what’s inside and whether there’s something inside that could be complicating for someone with food allergies," said Rep. Phillips. "It's not that complicated."
The ADINA Act has been introduced in the U.S. House of Representatives but has yet to receive a committee hearing. The Togal family encourages anyone who wishes to see this bill passed to contact their legislator.

