New Alzheimer’s drug by Lilly shows promise in clinical trial
Promising trial for Alzheimer's treatment
Eli Lilly last week announced the results of a Phase 3 trial on its experimental drug called Donanemab. The latest drug trial in a new class of Alzheimer’s drugs gives researchers and advocates renewed hope of slowing the progression of the brain disease.
MINNEAPOLIS (FOX 9) - The latest drug trial in a new class of Alzheimer’s drugs gives researchers and advocates renewed hope of slowing the progression of the brain disease.
Eli Lilly last week announced the results of a Phase 3 trial on its experimental drug called Donanemab. The drug attacks the amyloid proteins in the brain that lead to a buildup of plaque between neurons that weakens the ability of the neurons to communicate. The growth of these plaques along with tau protein strands leads to Alzheimer’s disease and dementia that robs a person of their memory, and eventually, their bodily functions leading to death.
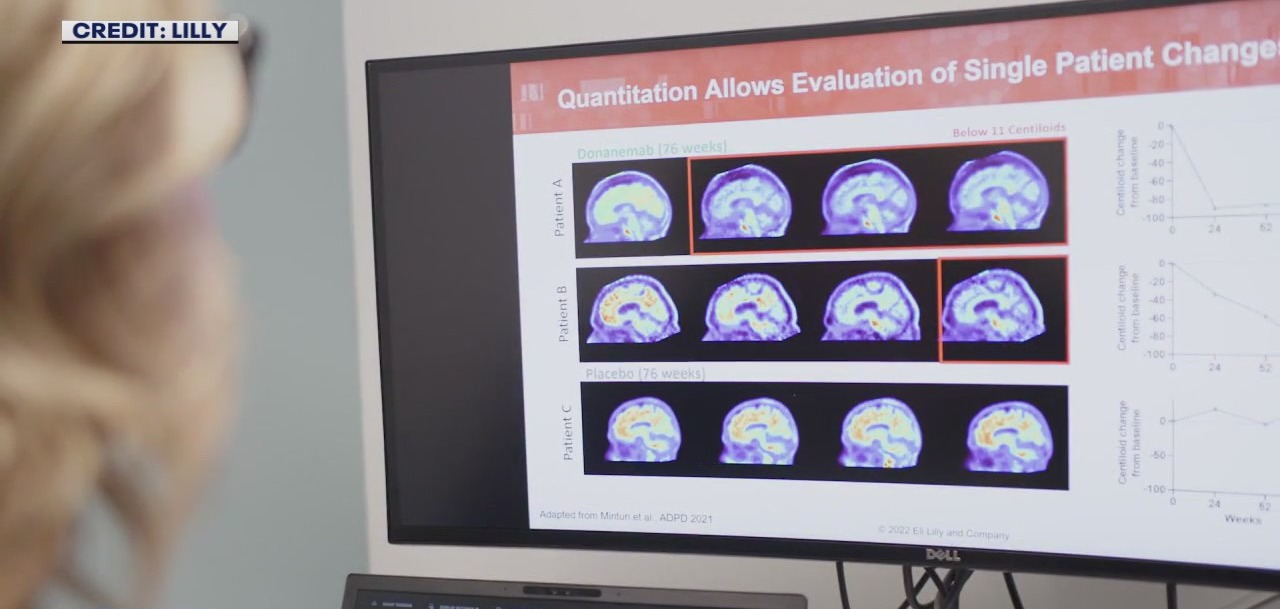
The research brief by Lilly says it conducted a randomized, double-blind, placebo-controlled study on 1,736 individuals. Some of the patients received Donanemab, the others received a dummy drug. Lilly says the study showed a 35% slowing of brain decline in the patients receiving Donanemab over 18 months. It also says researchers noticed 40% of patients had less decline in their ability to perform daily living activities. Additionally, 39% experienced a lower risk of progressing to the next level of Alzheimer’s disease.
"This is the third drug now that has been demonstrated to remove one of the proteins in the brain that causes Alzheimer’s disease," said Dr. Ronald Petersen, the director of the Alzheimer’s Disease Research Center at Mayo Clinic in Rochester, Minnesota.
"Donanemab is an antibody that goes into the bloodstream, circulates to the brain, grabs onto that protein called amyloid, and removes some of it from the brain," explained Petersen.
Earlier this year, Biogen in collaboration with Eisai received accelerated FDA approval for its Alzheimer’s drug, Lecanemab. The drug also targets the amyloid proteins where clinical trials showed it, too, mildly reduced the decline in brain function.
"So there are now three drugs that have been demonstrated to remove some of this amyloid protein from the brain and slow the clinical progression of the disease," said Petersen. "We haven’t had that prior to this, so this is very important to our patients."
The Alzheimer’s Association views the new drugs as giving families and patients hope.
"The results are so phenomenal that it really means in these early stages people will stay there longer, having more time, more time to create memories, more time to be independent, to stay active, engaged in their communities," said Susan Parriott, CEO of the Minnesota and North Dakota chapter of the Alzheimer’s Association. "It’s just a time to spend with their families, to make their own health care decisions."
These drugs are not a cure, and only offer the hope of slowing the progression of the disease in some patients. Dr. Petersen says in the case of Donanemab it could slow the progression of Alzheimer’s by roughly four and a half months over an 18-month period. This would allow people to possibly stay at their functional levels for a longer period of time.
"So this is the game changer for Alzheimer's disease in general, is that it should encourage people to go get diagnosed early because there is something they can do about it," said Parriott. "And if they get on these drugs before the dementia really sets in, what a difference it's going to make in their life."
Dr. Petersen says there are other drugs in the development stage to target the tau proteins in the brain as well.
"So I predict that in the future we'll use combination therapy to treat all kinds of disease," said Petersen. "Maybe a drug aimed at the amyloid protein, maybe another drug aimed at the tau protein. And the combination of these will provide an even greater benefit for our patients."
A big obstacle for patients at the moment is the coverage of these new drugs. Currently, the Centers for Medicaid and Medicare Services have not approved these drugs for coverage by Medicaid and Medicare.
"Most of the insurance companies follow right along. So if CMS says no, then the insurance companies will say no," explained Parriott who says the Veterans Administration has already granted approval for these drugs. "We're going back to CMS to say, listen, one of your own other government agencies says the results are good and the tests are good. Let's review this and get this approved."

Full interview: Dr. Ronald Petersen
Eli Lilly last week announced the results of a Phase 3 trial on its experimental drug called Donanemab. The drug attacks the amyloid proteins in the brain that lead to a buildup of plaque between neurons that weakens the ability of the neurons to communicate. The growth of these plaques along with tau protein strands leads to Alzheimer’s disease and dementia that robs a person of their memory, and eventually their bodily functions leading to death. Tim Blotz spoke with Dr. Ronald Petersen on the drug.

