RECALL: Depression medication Mirtazapine recalled due to label error on declared strength

RECALL: Depression Medication Mirtazapine Recalled Due to Label Error on Declared Strength
One lot of Mirtazapine tablets, used to treat major depressive disorder, was recalled due to a label error on its declared strength.
EAST WINDSOR, N.J. - One lot of Mirtazapine tablets, used to treat major depressive disorder, was recalled due to a label error on its declared strength.
Aurobindo Pharma USA, Inc. is voluntarily recalling lot number 03119002A3 of the Mirtazapine tablets on the consumer level, the company said in an announcement posted on the FDA’s website. The label error on its declared strength involved bottles labeled as Mirtazapine 7.5 mg possibly containing 15 mg tablets.
"Taking a higher dose than expected, may increase risk of sedation, agitation, increased reflexes, tremor, sweating, dilated pupils, gastrointestinal distress, nausea, constipation and more," the announcement said. "Unexpected levels of sedation in particular can contribute to falls in the elderly or motor vehicle accidents in adults."
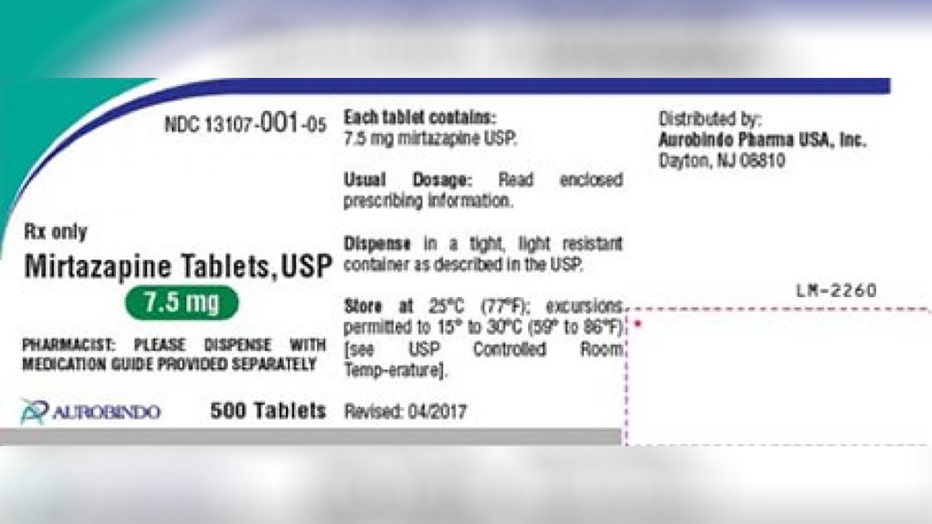
One lot of Mirtazapine tablets, used to treat major depressive disorder, was recalled due to a label error on its declared strength. (Photo: Aurobindo Pharma USA via FDA)
Mirtazapine tablets, packaged in 500 count bottles, can be identified by checking the product name, manufacturer details and batch or lot number on the bottle containing these products.
Aurobindo Pharma USA, Inc. is notifying its distributors by letter and is arranging for the return of all of the recalled product, according to the announcement. Distributors and retailers that have a product which is being recalled should return the bottle(s) to the place of purchase.
Consumers with medical questions regarding the recall or to report an adverse event can contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 Option 2 or pvg@aurobindousa.com.
Consumers should also contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
This story was reported from Los Angeles.

