Minnesota company's revolutionary hearing implant on FDA fast track
Minnesota company's revolutionary hearing implant on FDA fast track
A Minnesota-based medical device company is on the verge of transforming how hearing is improved. Envoy Medical's journey began decades ago as St. Croix Medical. Brent Lucas, the CEO, shared that the company was founded by an audiologist frustrated with traditional hearing aids. The company, now known as Envoy Medical, is making waves with its fully implanted hearing devices. FOX 9's Leah Beno has the story.
WHITE BEAR LAKE, Minn. (FOX 9) - A Minnesota-based medical device company is on the verge of transforming how hearing is improved.
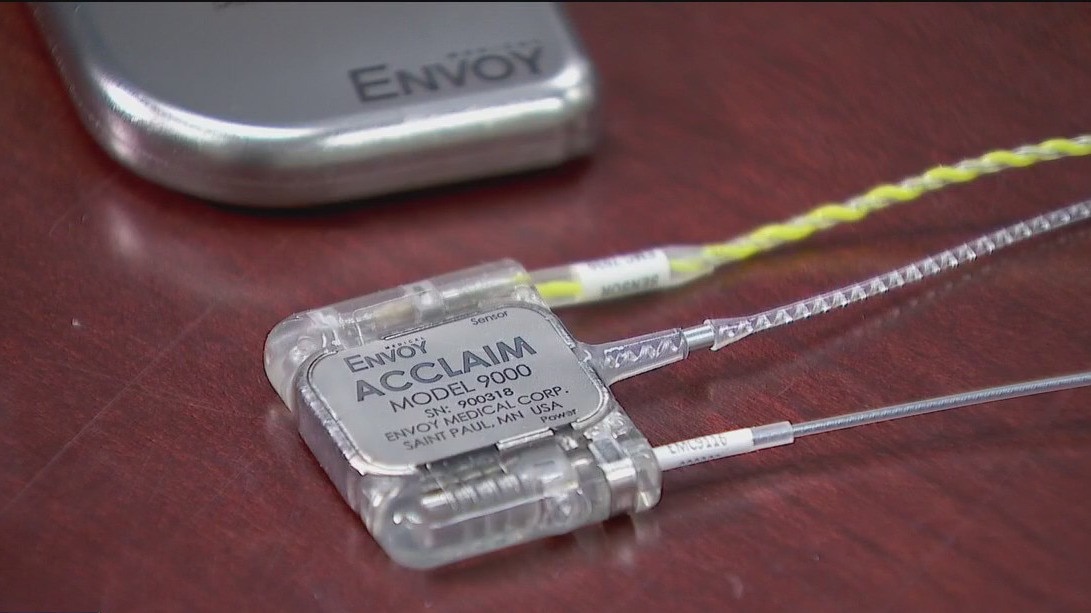
Envoy Medical's journey began decades ago as St. Croix Medical. Brent Lucas, the CEO, shared that the company was founded by an audiologist frustrated with traditional hearing aids. The company, now known as Envoy Medical, is making waves with its fully implanted hearing devices, Esteem and Acclaim, which differ from typical hearing aids and cochlear implants.
A new type of hearing aid
Patient Perspective:
The devices are unique because they don't rely on traditional microphones. Instead, they use the ear itself, allowing both devices to be completely implanted.
Craig Eggert, the first recipient of the Acclaim implant in October 2022, expressed initial nervousness but ultimately felt it was a life-changing decision. After years of using traditional hearing aids, Craig found the implanted device allowed him to engage in activities like hunting and swimming without the hassle of exterior devices.
Craig shared how his life had become smaller due to hearing challenges, avoiding loud environments and social gatherings. The implant has allowed him to return to these activities, significantly improving his quality of life.
FDA breakthrough designation
What's next:
The first version of the implant received FDA approval in 2010. Recently, the latest version was granted breakthrough device designation, putting it on the FDA fast track and expanding clinical trials from 10 to 46 patients. Lucas explained that this designation prioritizes the product, aiming to get it to patients quickly. Amy Pajula, who has implants in both ears, now works for Envoy Medical, addressing potential patient questions. She described taking the risk as a dream come true, despite knowing the challenges involved.
$250 million invested:
The journey has been costly, with investments from Medtronic executives, former Timberwolves owner Glen Taylor, and Brent's parents, who were among the first angel investors. Lucas noted that about $250 million has been invested over the past 30 years, emphasizing the potential upside. The timeline for insurance coverage for Envoy's products remains uncertain, but Lucas is hoping for FDA approval in 2027.
For more information, you can visit Envoy's website.

