Philips recalls 17M CPAP, BiPAP masks over magnets that could affect implanted devices

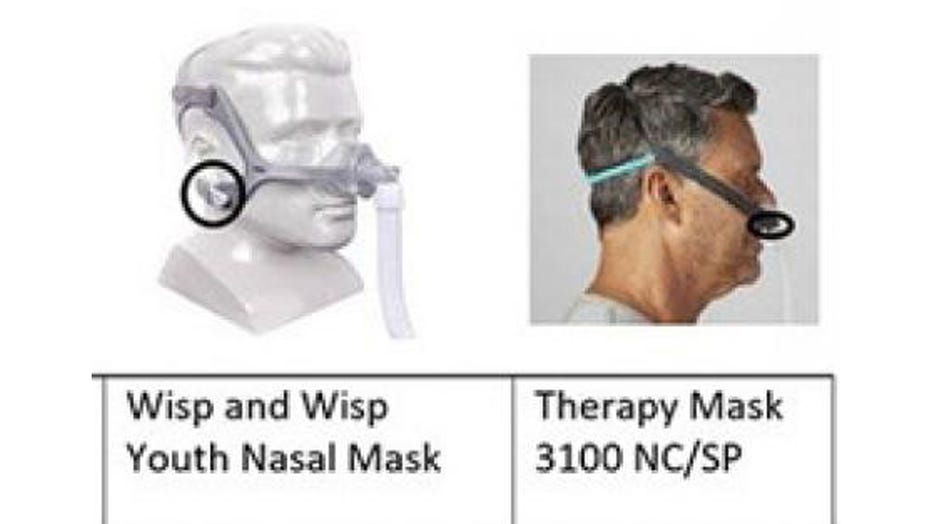
The impacted masks are pictured in provided photos. The magnet location is shown with black circles. (Credit: FDA)
WASHINGTON - Philips Respironics has recalled more than 17 million masks used for people with sleep apnea and other respiratory issues due to a safety issue with magnets that could affect certain implanted medical devices.
The recall was announced on Tuesday by the U.S. Food and Drug Administration after 14 serious injuries were reported, including pacemaker failure, arrhythmia, seizures, and irregular blood pressure.
The recalled masks are worn by a patient when using a bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP) machine and have magnetic headgear clips to hold them in place, officials said. They are for single-patient use in the home or multi-patient use in a hospital or other clinical settings.
The five mask types impacted by the recall are the DreamWisp, DreamWear, Amara View, Wisp, and Wisp Youth masks.
The impacted masks are pictured in provided photos. The magnet location is shown with black circles. (Credit: FDA)
The impacted masks are for patients weighing more than 66 pounds, except for the Wisp Youth Nasal Mask and Therapy Mask 3100 NC/SP, which are for patients aged 7 and older and weighing more than 40 pounds, the FDA said.
Phillips and the FDA said the masks have magnets that "can potentially cause injury or death if people who use them, or people near a person using a recalled mask, have certain implanted metallic medical devices or metallic objects in the body."
These devices include pacemakers, implantable cardioverter defibrillators, metallic stents (such as aneurysm, coronary, tracheobronchial, and biliary, neurostimulators (such as hypoglossal nerve stimulators) or magnetic metallic implants, electrodes, and valves placed in upper limbs, torso, neck, or head, officials said.
Other devices could include:
- Cerebral spinal fluid shunts (such as ventriculoperitoneal shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Ocular implants (such as glaucoma implants and retinal implants; intraocular lenses placed during cataract surgery are not impacted)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Implantable ports and pumps (such as insulin pumps)
- Metallic gastrointestinal clips
- Certain metallic joint replacements
- Devices labeled as Magnetic Resonance (MR) Unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
- Metallic splinters in the eye
- Metallic shrapnel in the body
Anyone with an implanted metallic device or object in the body listed above should stop using the recalled mask and switch to a non-magnetic mask if available, the FDA said.
"Ensure the recalled mask is kept at least 6 inches away from metallic medical implants, metallic objects in the body, or medical devices that can be impacted by the magnetic fields," the agency said.
In June 2021, certain Philips Respironics ventilators, CPAP, and BiPAP machines were recalled over concerns that a foam component could disintegrate and be inhaled by the user, possibly causing health issues, including toxic and carcinogenic effects.
Last month, Philips issued another recall for some of its respiratory machines due to a plastic component that could potentially be contaminated with a non-compatible material.
"This latest recall raises further safety concerns both for Philips devices already subject to a recall, as well as additional devices," Dr. Jeff Shuren director of the FDA’s Center for Devices and Radiological Health, said in the notice this week. "We strongly encourage providers and at-risk patients to review this important safety information and follow our recommended actions to reduce the potential for harm from these products."
This story was reported from Cincinnati.


